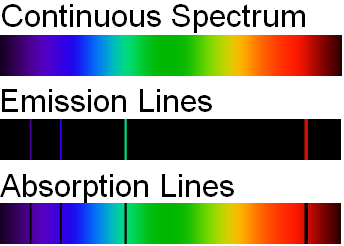
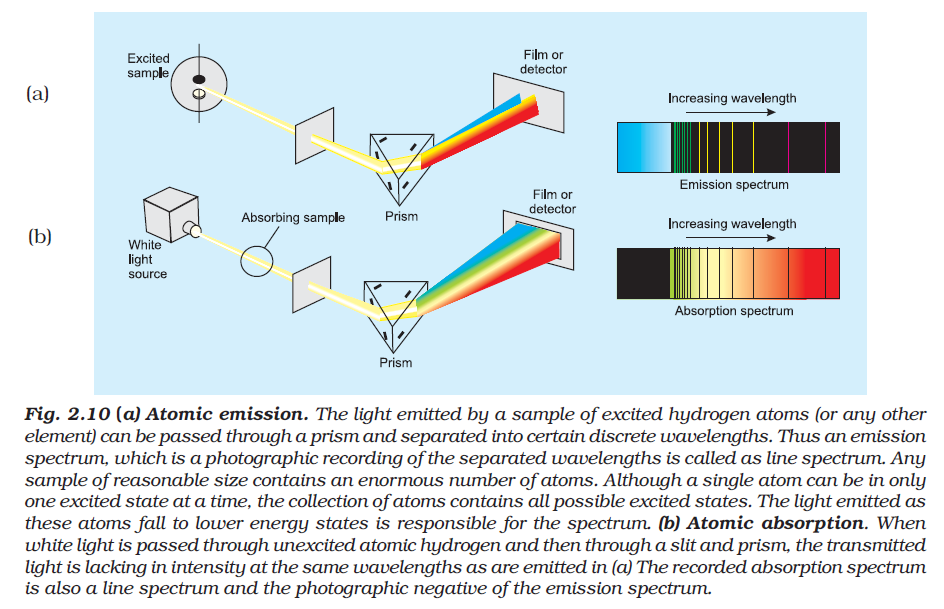
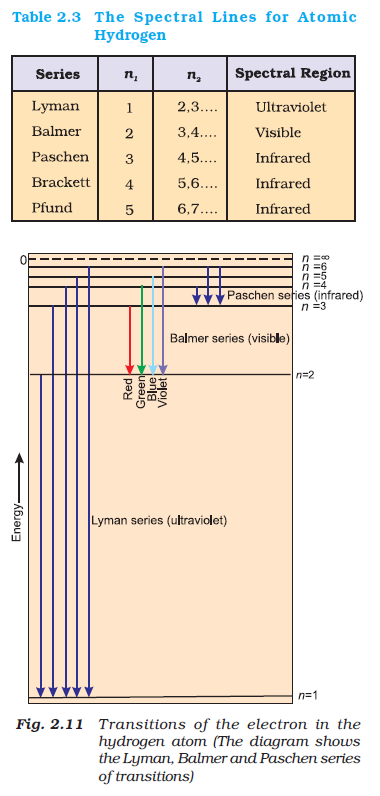
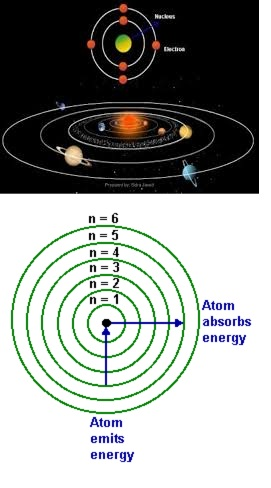
Dual Behaviour of EM Radiation :
Particle nature could explain black body radiation and photoelectric effect but could not explain phenomenon of interference and
diffraction.
So, it was accepted that light process both particles and wave - like properties i.e light has dual behaviour.
Some microscopic particles like electron also exhibit wave-particle duality.
diffraction.
So, it was accepted that light process both particles and wave - like properties i.e light has dual behaviour.
Some microscopic particles like electron also exhibit wave-particle duality.